
Medicines are manufactured in plants that meet very strict GMP rules. Thanks to this, we know that they have the right composition and do not contain toxins and impurities. And supplements? Not always.
By law, medicines must be produced in plants that meet Good Manufacturing Practice (GMP) standards. This is a set of rules that guarantees that medicines are made from the highest quality raw materials and in the cleanest conditions. It was created to minimize health risks at the production stage. Only the GMP system can guarantee that each drug package is of the same quality as the finished products sent for laboratory tests.
Such a high standard is not required by law for dietary supplements. Formally, they are treated as food, not medicines. In practice, this means that many supplements are manufactured in conditions that are far from pharmaceutical purity.
However, some companies , out of concern for quality and safety, outsource the supplement production process to GMP-certified plants. In these places, supplements are made in accordance with very strict principles of Good Manufacturing Practice, just like medicines.
What exactly do GMP rules apply to ?
- cleanliness of rooms and machines used to produce medicines;
- appropriate training for personnel of pharmaceutical plants (including occupational health and safety);
- purity of the composition of raw materials and products based on them. Contamination with substances, e.g. from neighboring production, may cause not only health damage, but also death;
- labeling packaging correctly to avoid patients receiving the wrong medicine;
- compliance of the composition with the contents of the drug packaging. Insufficient or too much active substance may render the treatment ineffective. It may also lead to side effects.
There are detailed written instructions for each production stage. Each GMP-certified plant has a system to ensure strict compliance. It works at every stage and every time the product is manufactured. Special inspections are also carried out in such plants to check quality standards according to GMP .
It is sometimes said that this entire system comes down to the "5 P": personnel, products, processes, procedures, and rooms and equipment . Each of these components has a direct impact on the quality of the final product .
-
Staff
The plant should employ an appropriate number of suitably qualified and experienced employees. Each of them must undergo GMP training and fully understand their roles and responsibilities.
-
Products
All products must be constantly tested. Manufacturers should ensure the purity of raw materials and other materials. They must be protected from contamination at every stage. All labels for marking containers, devices and rooms must be legible and unambiguous.
-
Processes
Processes should be properly documented, clear and consistent. Employees must be well versed in them. Regular assessment should be carried out to ensure that processes are followed correctly. Each step of the process must be documented.
-
Procedures
Procedures, i.e. guidelines that apply to individual operations. Procedures must be well known to all employees and consistently followed. Any deviations from standard procedures must be reported immediately. Each such case should be thoroughly investigated.
-
Rooms and equipment
Production rooms should be kept as clean as possible. The goal is to avoid cross-contamination as well as accidents. Production halls and production warehouses must have appropriate air temperature, humidity, lighting and ventilation. All equipment must be properly calibrated, cleaned and maintained. Its arrangement and design must minimize the risk of error.
In addition to the above-mentioned issues, GMP also regulates the storage and transport of raw materials and finished products. GMP rules also apply to water supply to plants , sewage and waste management, the complaint process and product recalls . It is a very complex system with a large number of specific requirements.
In practice, all this is needed to ensure that the finished product (medicine or dietary supplement):
- it contains the amount of active substances as planned at the beginning, and the composition is consistent with the label;
- it is free from contaminants, it does not contain anything that is not on the label;
- is stored in the plant in a way that does not affect its properties.
And even though the supplement is not formally a medicine, everyone would probably prefer that the capsules or tablets they take every day meet the above strict conditions and are as safe as possible.
Bibliography:
- “Medicines: Good manufacturing practices”, World Health Organization. who.org, November 20, 2015
- “Good manufacturing practice”, European Medicines Agency, ema.europa.eu, accessed January 10, 2022.
- “GMP: Good Manufacturing Practices”, safetyculture.com, December 15, 2021
- Duszyńska M., “GMP - Good Manufacturing Practice”, laboratories.net, accessed January 10, 2022.

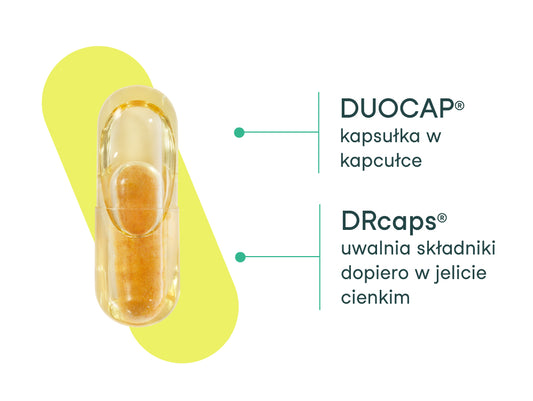
What makes our capsule stand out?
The nikalab capsule impresses not only with its appearance, but also with its operation. We used two innovative...







